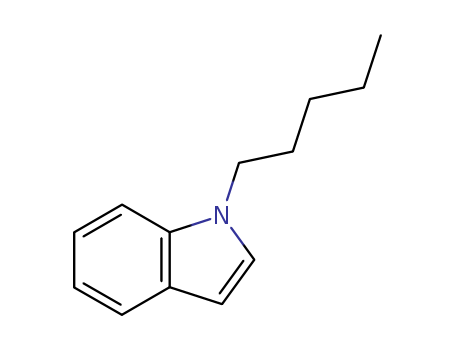
CasNo: 59529-21-4
Molecular Formula: C13H17N
|
Uses |
1-Pentyl-1H-indole is a chemical compound that belongs to the class of compounds known as indoles. 1-Pentyl-1H-indole, or AM-2201, is a synthetic cannabinoid that was initially developed as a research chemical for studying the endocannabinoid system. It is structurally related to cannabinoids found in the cannabis plant and was created for use in scientific research to better understand the interaction of cannabinoids with the human body. |
InChI:InChI=1/C8H5BrFN/c9-4-7-3-8(10)2-1-6(7)5-11/h1-3H,4H2
Purpose: This work reports the synthesis...
Five-carbon (C5) structural units are th...
Synthetic cannabinoid receptor agonists ...
The functionalization of C?H bonds with ...
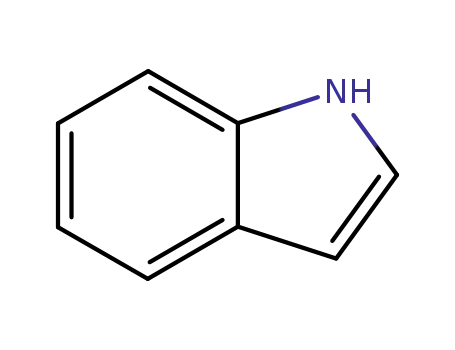
indole

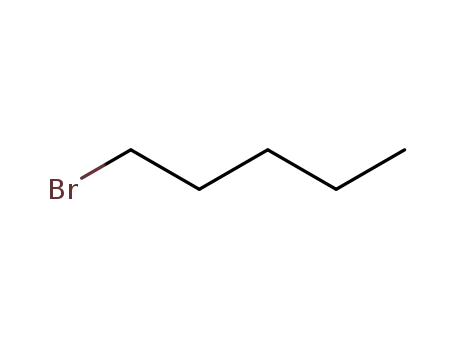
1-Bromopentane

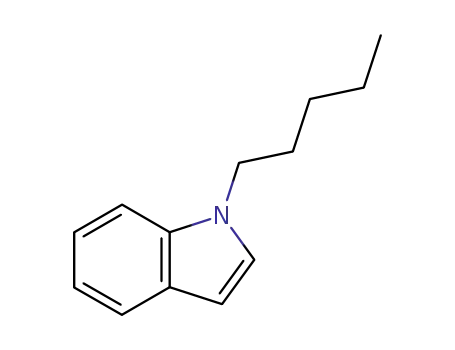
1-pentyl-1H-indole
| Conditions | Yield |
|---|---|
|
indole; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 0 - 20 ℃; for 0.5h;
1-Bromopentane; In N,N-dimethyl-formamide; mineral oil; at 0 - 20 ℃;
|
100% |
|
indole; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 5 - 20 ℃; Inert atmosphere;
1-Bromopentane; In N,N-dimethyl-formamide; mineral oil; at 5 - 20 ℃; for 16h; Inert atmosphere;
|
95% |
|
With potassium hydroxide; tetrabutylammomium bromide; potassium carbonate; for 0.0075h; microwave irradiation (300 W);
|
86% |
|
indole; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 20 ℃; for 0.166667h; Inert atmosphere;
1-Bromopentane; In N,N-dimethyl-formamide; mineral oil; at 0 - 20 ℃; Inert atmosphere;
|
80% |
|
With sodium hydride; Yield given. Multistep reaction; 1.) DMF, 90 min, 2.) 50-55 deg C, 36 h;
|
|
|
indole; With sodium hydride; In N,N-dimethyl-formamide; at 0 ℃; for 0.5h;
1-Bromopentane; In N,N-dimethyl-formamide; at 0 ℃;
|
|
|
indole; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; for 0.166667h; Cooling with ice; Inert atmosphere;
1-Bromopentane; In N,N-dimethyl-formamide; mineral oil; at 20 ℃; for 1h; Cooling with ice; Inert atmosphere;
|
|
|
indole; With sodium hydride; In N,N-dimethyl-formamide; at 0 - 20 ℃; for 0.5h; Inert atmosphere;
1-Bromopentane; In N,N-dimethyl-formamide; at 0 - 20 ℃; for 1h; Inert atmosphere;
|
|
|
indole; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 0 - 20 ℃; for 0.5h;
1-Bromopentane; In N,N-dimethyl-formamide; mineral oil; at 0 - 20 ℃;
|

indole

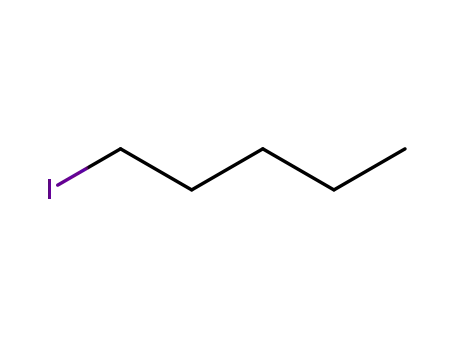
amyl iodide


1-pentyl-1H-indole
| Conditions | Yield |
|---|---|
|
With triethylamine; In acetonitrile; at 80 ℃; for 16h;
|
75% |

indole
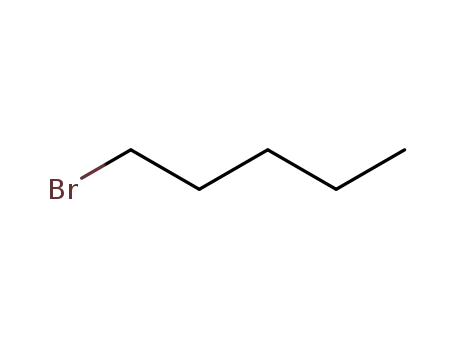
1-Bromopentane
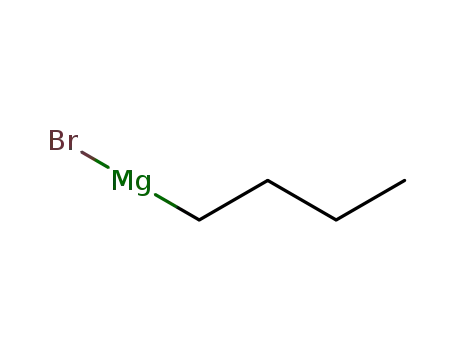
n-butyl magnesium bromide
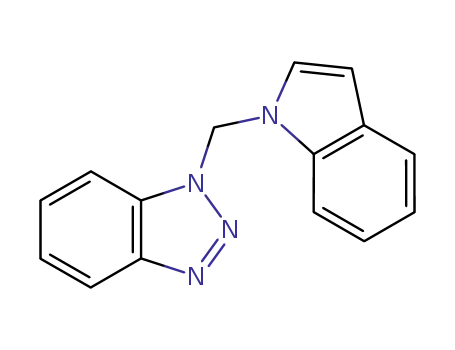
1-(1H-benzotriazol-1-yl-methyl)-1H-indole
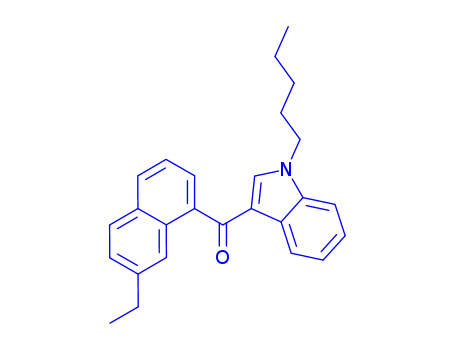
(7-ethyl-1-naphthalenyl)(1-pentyl-1H-indol-3-yl)methanone
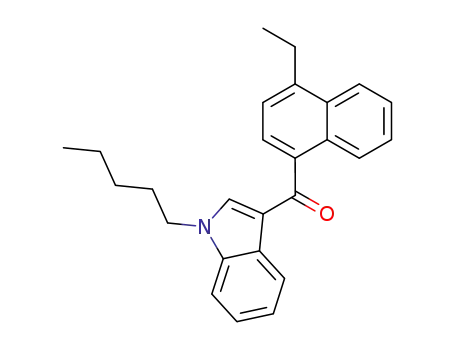
JWH-210
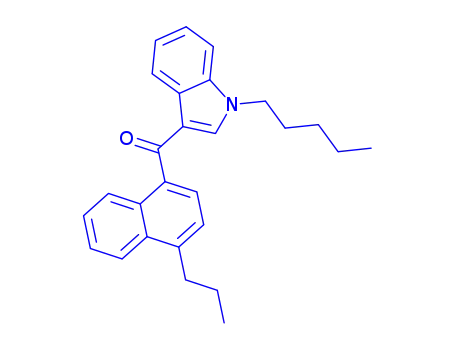
(1-pentyl-1H-indol-3-yl)(4-propyl-1-naphthalenyl)methanone
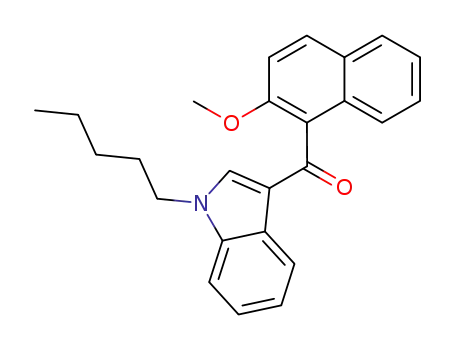
(2-methoxy-1-naphthalenyl)(1-pentyl-1H-indol-3-yl)methanone