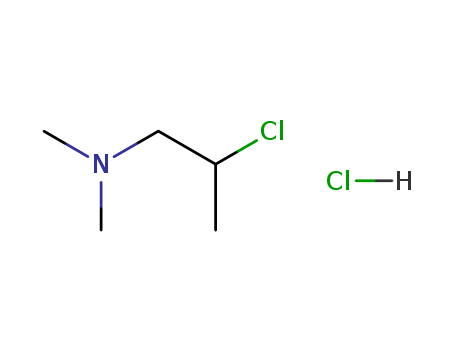
CasNo: 4584-49-0
Molecular Formula: C5H13Cl2N
Appearance: Colourless to pale beige crystalline powder
|
Chemical Properties |
Colourless to pale beige crystalline powder |
|
Uses |
2-Chloro-N,N-dimethylpropylamine hydrochloride (DMIC) is used as intermediate for the syntheses of pharmaceuticals (e.g. isothipendyl, methadone and promethazine) |
|
General Description |
Off-white chunky solid. |
|
Air & Water Reactions |
2-Dimethylaminoisopropyl chloride hydrochloride is hygroscopic. Soluble in water. |
|
Reactivity Profile |
2-Dimethylaminoisopropyl chloride hydrochloride is incompatible with strong oxidizing agents. . Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. |
|
Fire Hazard |
Flash point data for 2-Dimethylaminoisopropyl chloride hydrochloride are not available; however, 2-Dimethylaminoisopropyl chloride hydrochloride is probably combustible. |
|
Synthesis |
Following the procedure of Schultz and Sprague, J. Am. Chem. Soc., 70, 48 (1948), a solution containing 3.77 g. of 1-dimethylamino-2-propanol and 10 ml. of chloroform was cooled with stirring to a temperature of about 0° C. A solution of 5.72 g. of freshly distilled thionylchloride in 2 ml. of chloroform was added thereto. The reaction mixture was allowed to come to ambient temperature over a period of about 30 minutes and was then heated to refluxing temperature for an additional 30 minutes. The precipitated material redissolved on heating. 1-Dimethylamino-2-chloropropane hydrochloride began to crystallize from the boiling solvent. The reaction mixture was cooled, diluted with ether and filtered. The reaction product comprising 1-dimethylamino-2-chloropropane hydrochloride weighed about 5.5 g. (95 percent yield). Recrystallization yielded purified 1-dimethylamino-2-chloropropane hydrochloride melting at 192°-194° C. |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C5H12ClN/c1-5(6)4-7(2)3/h5H,4H2,1-3H3/p+1/t5-/m0/s1
The systemic investigation of the struct...
The invention relates to the compounds o...
The 2-chloro-1-dialkylamino-propanes 4a-...
Alkylation of diphenylacetonitrile in me...
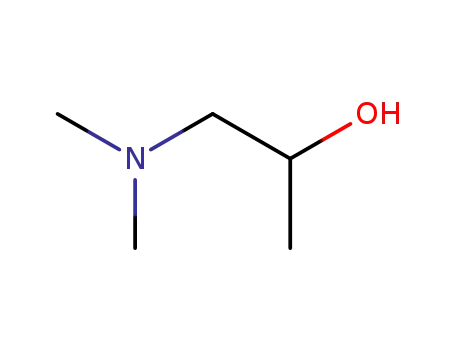
1-methyl-2-N,N-dimethylaminoethanol

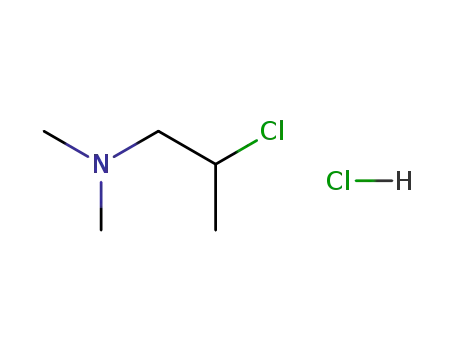
(2-chloropropyl)dimethylamine hydrochloride
| Conditions | Yield |
|---|---|
|
With
thionyl chloride;
In
chloroform;
at 0 - 20 ℃;
for 1h;
Reflux;
|
95% |
|
With
thionyl chloride;
In
chloroform;
|
95% |
|
With
thionyl chloride;
In
chloroform;
Reflux;
Cooling with ice;
|
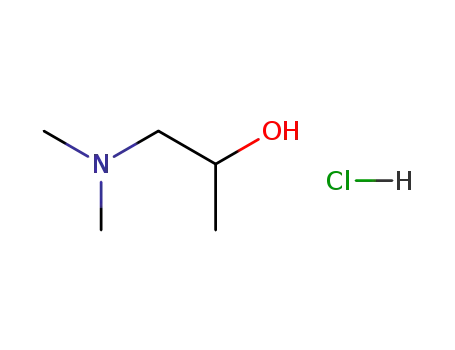
1-dimethylamino-2-propanol hydrochloride


(2-chloropropyl)dimethylamine hydrochloride
| Conditions | Yield |
|---|---|
|
With
thionyl chloride;
at 60 ℃;
for 24h;
|

1-methyl-2-N,N-dimethylaminoethanol
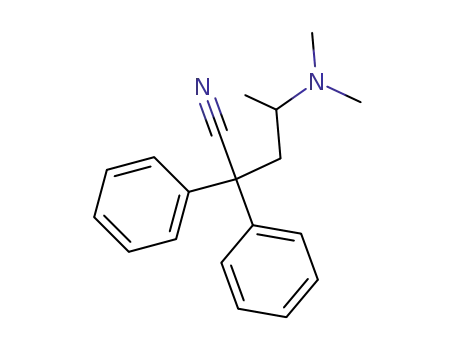
(R,S)-2,2-diphenyl-4-dimethylaminopentanenitrile
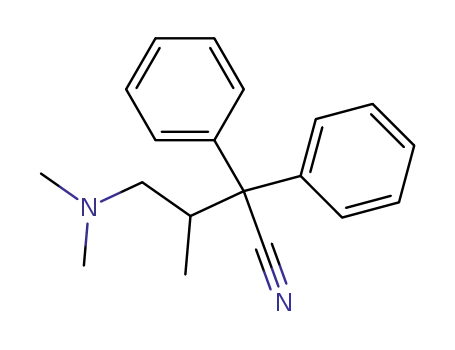
2,2-diphenyl-3-methyl-4-(dimethylamino)-butyronitrile
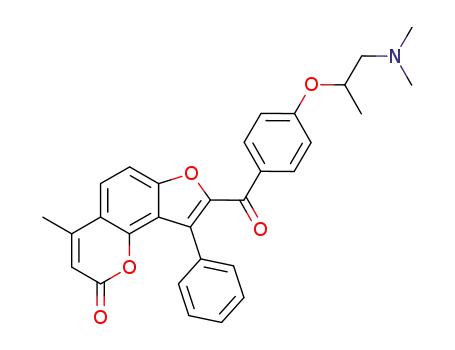
8-[4-(2-Dimethylamino-1-methyl-ethoxy)-benzoyl]-4-methyl-9-phenyl-furo[2,3-h]chromen-2-one
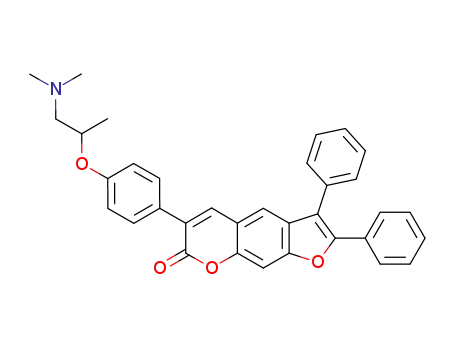
6-[4-(2-Dimethylamino-1-methyl-ethoxy)-phenyl]-2,3-diphenyl-furo[3,2-g]chromen-7-one