CasNo: 14252-80-3
Molecular Formula: C18H28N2O.ClH
Appearance: white solid
|
Chemical Properties |
White Solid |
|
Uses |
Bupivacaine HCl is an FDA-approved local anesthetic commonly used in medical and dental procedures in the United States. When administered by trained healthcare professionals following recommended guidelines and safety precautions, Bupivacaine HCl is generally considered safe and effective for providing localized anesthesia. |
|
Brand name |
Marcaine (Hospira); Sensorcaine (AstraZeneca). |
|
Biological Functions |
Bupivacaine hydrochloride (Marcaine, Sensorcaine) has particularly long action, and some nerve blocks last more than 24 hours; this is often an advantage for postoperative analgesia. Its use for epidural anesthesia in obstetrics has attracted interest because it can relieve the pain of labor at concentrations as low as 0.125% while permitting some motor activity of abdominal muscles to aid in expelling the fetus. The lower concentration minimizes the possibility of cardiac toxicity. Fetal drug concentrations remain low, and drug-induced neurobehavioral changes are not observed in the newborn. Bupivacaine also is approved for spinal anesthesia and is approximately four times more potent and more toxic than mepivacaine and lidocaine. It can be used with or without epinephrine. |
InChI:InChI=1S/C18H28N2O.ClH/c1-4-5-12-20-13-7-6-11-16(20)18(21)19-17-14(2)9-8-10-15(17)3;/h8-10,16H,4-7,11-13H2,1-3H3,(H,19,21);1H
Design of water-soluble prodrugs may con...
Liposome bupivacaine was associated with a significant reduction in opioid use (12 mg vs 19 mg; p < 0.0001) and incidence of ORAEs (20% vs 36%; p < 0.0001), compared with bupivacaine HCl.
With DepoFoam bupivacaine 532-mg, differences in NRS-R scores reached statistical significance (P < 0.05) vs bupivacaine HCl on Days 1 and 5 and mean AUC NRS-R scores were significantly lower through Days 2–5; a dose–response trend was demonstrated.
Herein we report a convenient, fast, and...

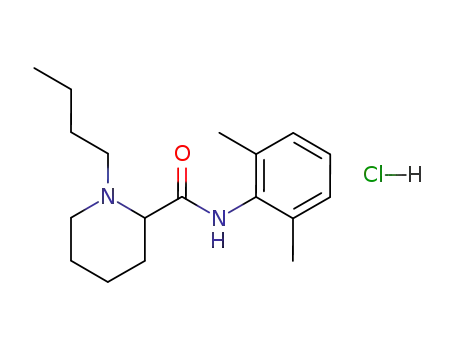
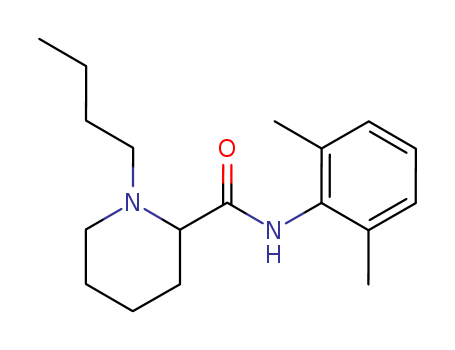
bupivacaine hydrochloride
| Conditions | Yield |
|---|---|
|
With tetrabutylammomium bromide; potassium carbonate; In water; toluene; at 80 - 85 ℃; for 8.5h;
With hydrogenchloride; In isopropyl alcohol; acetone; toluene; at 40 ℃;
|
92% |
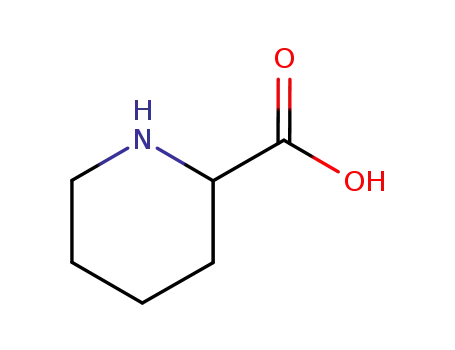
pipecolic Acid


bupivacaine hydrochloride
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 4 steps
1.1: sodium hydroxide / water / 12 h / 20 °C
2.1: N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate / N,N-dimethyl-formamide / 1 h / 20 °C
2.2: 18 h / 20 °C
3.1: palladium on activated charcoal; hydrogen / methanol / 0.5 h / 50 °C / Autoclave
4.1: N,N-dimethyl-formamide / 12 h / 20 - 80 °C
4.2: 20 °C
With palladium on activated charcoal; hydrogen; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate; sodium hydroxide; In methanol; water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 4 steps
1.1: toluene-4-sulfonic acid / ethanol / 1 h / 20 °C
1.2: 6 h / 30 - 40 °C
2.1: 1,1'-carbonyldiimidazole / dimethyl sulfoxide / 1 h / 20 °C
2.2: 8 h / 130 - 140 °C
3.1: ethanol; water / 1 h / Reflux
4.1: ammonia / methanol / 2 h
With ammonia; toluene-4-sulfonic acid; 1,1'-carbonyldiimidazole; In methanol; ethanol; water; dimethyl sulfoxide;
|
|
|
Multi-step reaction with 3 steps
1.1: hydrogenchloride / toluene / 1 h / 20 °C
1.2: 7 h / 55 °C
2.1: toluene / 2 h / 60 °C
3.1: potassium carbonate / N,N-dimethyl-formamide / 1.5 h / 90 °C
With hydrogenchloride; potassium carbonate; In N,N-dimethyl-formamide; toluene;
|
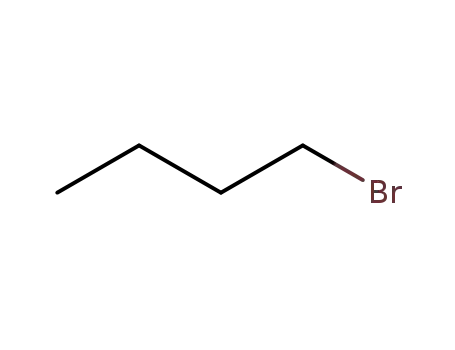
1-bromo-butane

bupivacaine hydrochloride
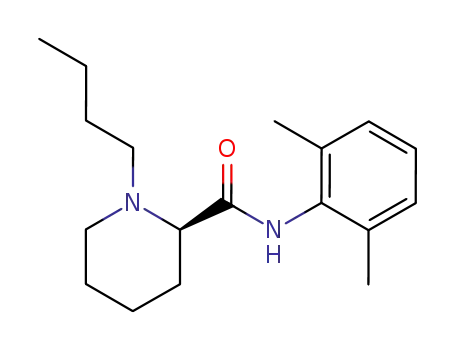
(+)-Bupivacaine
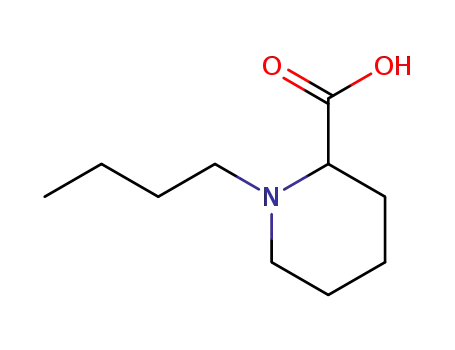
racemic 1-butylpiperidine-2-carboxylic acid

(+)-Bupivacaine
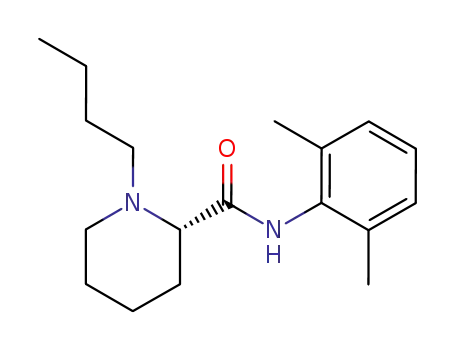
levobupivacaine