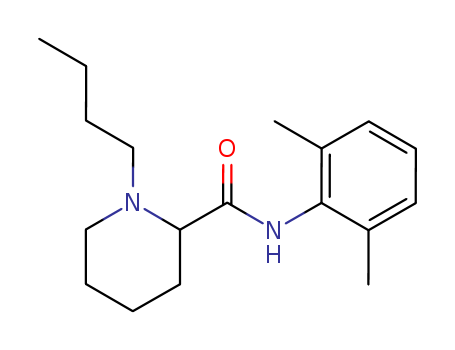
CasNo: 2180-92-9
Molecular Formula: C18H28N2O
|
Originator |
Carbostesin,Astra,W. Germany,1967 |
|
Uses |
Bupivacaine, including Bupivacaine Base, is an FDA-approved local anesthetic commonly used in medical and dental procedures in the United States. When administered by trained healthcare professionals following recommended guidelines and safety precautions, Bupivacaine is generally considered safe and effective for providing localized anesthesia. |
|
Brand name |
Marcaine (Hospira); Sensorcaine (AstraZeneca). |
|
Therapeutic Function |
Local anesthetic |
|
Mechanism of action |
Bupivacaine is a local anaesthetic containing a chiral centre and adopts dextro and laevo forms. The enantiopure l form is less cardio- and neurotoxic and has an equivalent potency to the racemic mixture; therefore levobupivacaine is often preferred to reduce the potential for toxicity. Stereoselectivity describes the differences in response at a given receptor for the different enantiomers (such as the response discussed for S(+) ketamine). The opioid and NMDA receptors also exhibit stereoselectivity. |
|
Side effects |
Common side effects of bupivacaine include:weakness, long-lasting numbness or tingling;feeling restless or drowsy;tremors;headache, blurred vision;fast or slow heartbeats;breathing problems;chills or shivering;back pain; nausea, vomiting. |
InChI:InChI=1/C18H28N2O/c1-4-5-12-20-13-7-6-11-16(20)18(21)19-17-14(2)9-8-10-15(17)3/h8-10,16H,4-7,11-13H2,1-3H3,(H,19,21)
The invention provides hydrohphobic drug...
This article provides an overview of the efficacy profile of liposome bupivacaine based on Phase II and Phase III data from 10 randomized, double-blind, controlled, single-dose wound infiltration studies in patients undergoing hernia repair, total knee arthroplasty, hemorrhoidectomy, breast augmentation, or bunionectomy.
Herein we report a convenient, fast, and...
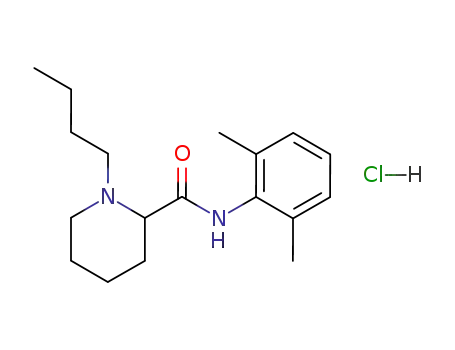
bupivacaine hydrochloride

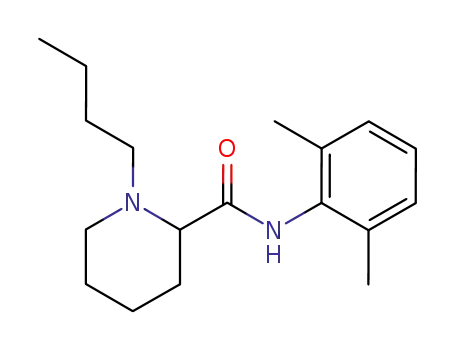
racemic bupivacaine
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In water; pH=9.5;
|
93% |
|
With sodium hydroxide; In water; at 20 ℃; pH=10.8; Alkaline conditions;
|
|
|
With sodium hydroxide; In water;
|
|
|
Multi-step reaction with 2 steps
1.1: sodium hydroxide / tert-butyl methyl ether; water / 45 °C
1.2: 75 °C
2.1: water / ethylene glycol / 9 h / 138 °C
With water; sodium hydroxide; In tert-butyl methyl ether; water; ethylene glycol;
|
|
|
With sodium hydrogencarbonate; In water; acetone; at 20 ℃; pH=7;
|
23.9 g |
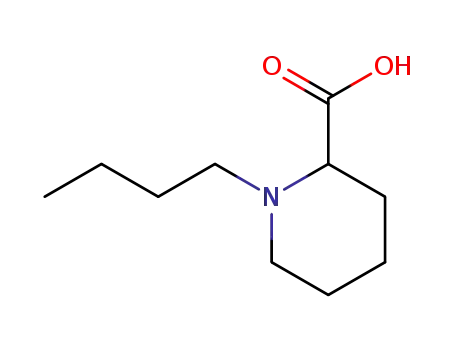
racemic 1-butylpiperidine-2-carboxylic acid

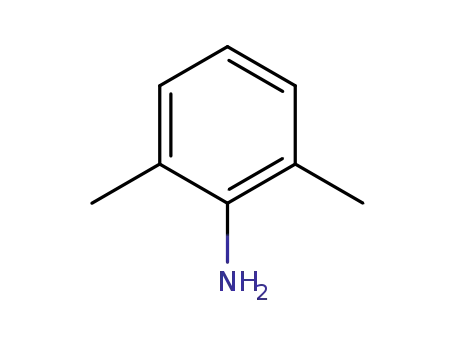
2,6-dimethylaniline


racemic bupivacaine
| Conditions | Yield |
|---|---|
|
racemic 1-butylpiperidine-2-carboxylic acid; With 1,1'-carbonyldiimidazole; In dimethyl sulfoxide; at 20 ℃; for 1h;
2,6-dimethylaniline; In dimethyl sulfoxide; at 130 - 140 ℃; for 8h;
|
79.9% |
|
racemic 1-butylpiperidine-2-carboxylic acid; With 1,1'-carbonyldiimidazole; In dimethyl sulfoxide; at 20 ℃; for 1h;
2,6-dimethylaniline; In dimethyl sulfoxide; at 130 - 140 ℃; for 8h;
|
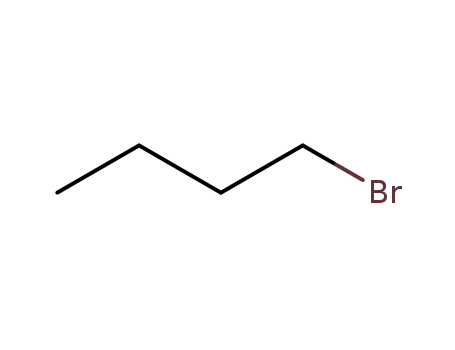
1-bromo-butane

bupivacaine hydrochloride
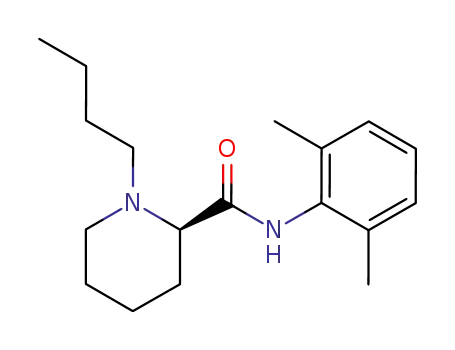
(+)-Bupivacaine

racemic 1-butylpiperidine-2-carboxylic acid

(+)-Bupivacaine
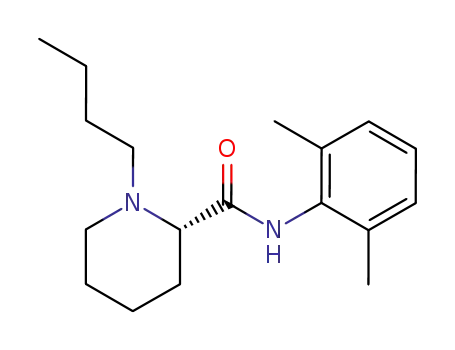
levobupivacaine