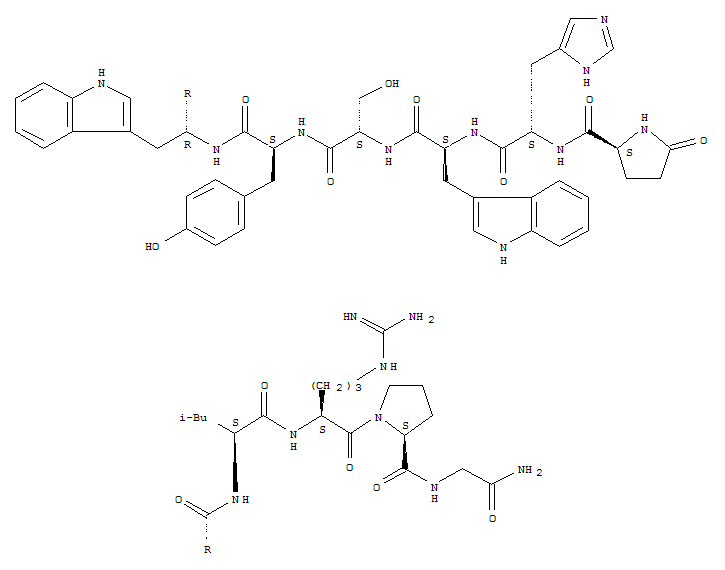
CasNo: 57773-63-4
Molecular Formula: C64H82N18O13
Appearance: powder
|
Antineoplastic drug |
The active ingredient of triptorelin is a synthetic gonadotropin-releasing hormone (GnRH) analogue, which is also known as decapeptyl, diphereline, triptorelin acetate, gonadorelin. It is an antineoplastic drug, whose pharmacological effects are same with goserelin, buserelin, and its structure is modified by natural molecular structure of the sixth L-glycine that is replaced by D-tryptophan to promote efficiency and prolong the plasmic half-life time, as well as enhance drug efficacy. After intramuscular injection, it initially stimulates the pituitary gland to release luteinizing growth hormone (LH) and follicle-stimulating hormone (FSH). When the pituitary over a long period of stimulation will be into the refractory period, that reduced the release of gonadotropin, leading to sex hormones reduction to castrate levels. These effects are reversible. Subcutaneous administration is rapidly absorbed, and Tmax is 15min, and the effect is maximum at 1h. The intramuscular injection of sustained release formulations can maintain the efficacy reaching 28d or more. It Clinically uses for the treatment of endometriosis, hormone-dependent prostate cancer, breast cancer, precocious puberty, as well as it may also be used as assisted reproductive technology. The main side effects of triptorelin are as follows: In the early stage of treatment of male, it can cause urinary disorders, breast swelling and pain, bone pain, and it may also cause hot flashes, loss of libido and impotence. It rarely cause gynecomastia, testicular atrophy, and sleep disorders. In the first week of treatment for children, girls will occur small amount of vaginal bleeding, which can be corrected by short-term additional therapy. During the treatment of women, hot flashes, bleeding or bleeding spots, vaginal dryness, headaches and weakness can occur. It can cause abdominal and (or) pelvic pain when it uses combination with gonadotropin. Since the concentration of estrogen reduces to the level of post-menopause, it can cause slight loss of trabecular bone matrix. Generally, after cessation of treatment six to nine months the adverse reations can return to normal. There may be irreversible amenorrhea after treatment in patients with endometriosis. |
|
Description |
Decapeptyl is a modified (D-Trp6) LH-RH. Like recently marketed buserelin and leuprolide (I), it is useful in achieving medical castration in the treatment of advanced prostate cancer. |
|
Originator |
Tulane Univ. (USA) |
|
Uses |
promoting ovulation |
|
Definition |
ChEBI: An oligopeptide comprising pyroglutamyl, histidyl, tryptophyl, seryl, tyrosyl, D-tryptophyl, leucyl, arginyl, prolyl and glycinamide residues joined in sequence. It is an agonist analogue of gonadotropin-releasing hormone. |
|
Brand name |
Trelstar (Watson). |
|
Biochem/physiol Actions |
Potent LH-RH agonist with enhanced biological activity due to its slower rate of degradation. Like [D-Lys6]-LH-RH, the D-Trp6 analog has been shown to be effective against cancers expressing the LH-RH receptor. However, unlike the D-Lys6 analog, it is generally used in the unconjugated form. |
|
Clinical Use |
Triptorelin pamoate is another superagonist of GnRH, which like nafarelin acetate contains only a single amino acid substitution (D-Trp6 for Gly6) when compared to the natural hormone. In the treatment of advanced prostate cancer, it is important to reduce serum testosterone levels to very low levels, which can be achieved surgically by orchiectomy. When this surgical method is unacceptable to the patient, an alternative approach is “chemical castration,” which can be achieved by use of estrogen therapy, leuprolide,goserelin or histrelin acetates, and now, triptorelin pamoate. This product is available for IM depot injection (monthly or every 3 months), wherein serum testosterone concentration drops to a level generally seen in surgically castrated men. |
|
Drug interactions |
Potentially hazardous interactions with other drugs None known |
|
Metabolism |
The metabolism of triptorelin in humans is unknown, but it is thought to be hydrolysed in the plasma and excreted in the urine as inactive metabolites. |
InChI:InChI=1/C42H59N11O9.C22H25N7O4.C2H4O2/c1-24(2)19-31(37(58)50-30(9-5-16-46-42(44)45)41(62)53-17-6-10-34(53)40(61)48-23-35(43)56)51-39(60)33(21-26-22-47-29-8-4-3-7-28(26)29)52-38(59)32(49-36(57)15-18-54)20-25-11-13-27(55)14-12-25;23-20(31)17(7-12-9-25-15-4-2-1-3-14(12)15)28-22(33)18(8-13-10-24-11-26-13)29-21(32)16-5-6-19(30)27-16;1-2(3)4/h3-4,7-8,11-14,22,24,30-34,47,54-55H,5-6,9-10,15-21,23H2,1-2H3,(H2,43,56)(H,48,61)(H,49,57)(H,50,58)(H,51,60)(H,52,59)(H4,44,45,46);1-4,9-11,16-18,25H,5-8H2,(H2,23,31)(H,24,26)(H,27,30)(H,28,33)(H,29,32);1H3,(H,3,4)/t30-,31-,32-,33+,34-;16-,17-,18-;/m00./s1