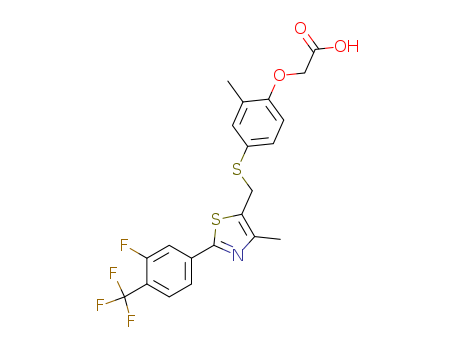
CasNo: 317318-84-6
Molecular Formula: C21H17 F4 N O3 S2
Appearance: White to off-white solid
|
Description |
GW0742 is a PPARδ/β agonist very similar in molecular structure to GW501516 (Cardarine) — with the exception of one atom. It is often mentioned in comparison with Cardarine. This drug is mostly used by athletes to improve their stamina. The fat affecting properties of GW0742 also make it a good promoter of energy in the body. Additionally, this drug helps increase the oxidative abilities of your body muscles. |
|
Chemical Properties |
Light Yellow Solid |
|
Uses |
GW0742 is a small molecule agonist of the human Peroxisome Proliferator-Activated Recept δ (PPAR δ). It shows an EC50 of 1.1 nM against PPAR δ with 100-fold selectivity over the other human subtypes. |
|
Biological Activity |
Potent and highly selective PPAR δ agonist. EC 50 values are 0.001, 1.1 and 2 μ M for transactivation of human PPAR δ , - α , and - γ receptors respectively. Neuroprotective in rat cerebellar granule neuronal cultures after brief (12-hour) exposure but exhibits inherent toxicity after prolonged (48-hour) incubation. Increases rate of fatty acid oxidation and protects against ischemia/reperfusion injury in neonatal and adult cardiomyocytes. |
InChI:InChI=1/C21H17F4NO3S2/c1-11-7-14(4-6-17(11)29-9-19(27)28)30-10-18-12(2)26-20(31-18)13-3-5-15(16(22)8-13)21(23,24)25/h3-8H,9-10H2,1-2H3,(H,27,28)
We describe the parallel synthesis of no...
Methods or prevention or treatment of di...
We report the synthesis and biological a...
Compounds of Formula (I) are disclosed. ...
C25H25F4NO3S2

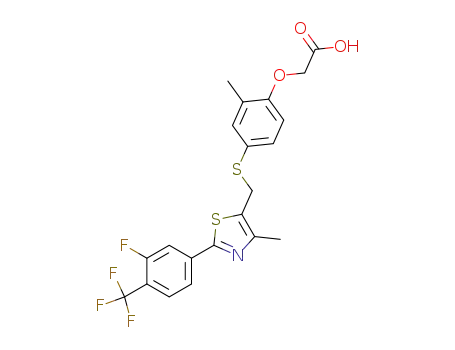
GW 0742
| Conditions | Yield |
|---|---|
|
With
trifluoroacetic acid;
In
dichloromethane;
at 20 ℃;
for 1h;
|
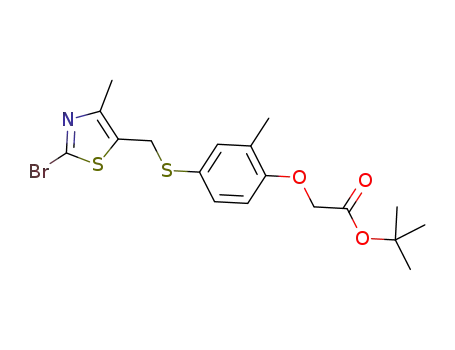
tert-butyl 2-(4-(((2-bromo-4-methylthiazol-5-yl)methyl)thio)-2-methylphenoxy)acetate


GW 0742
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: sodium carbonate / 1,2-dimethoxyethane / 0.5 h / 150 °C / Inert atmosphere; Sealed tube; Microwave irradiation
2: trifluoroacetic acid / dichloromethane / 1 h / 20 °C
With
sodium carbonate; trifluoroacetic acid;
In
1,2-dimethoxyethane; dichloromethane;
1: |Suzuki Coupling;
|

4-hydroxy-3-methylphenyl methyl ketone
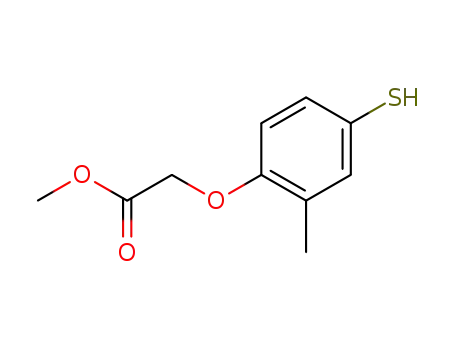
(4-mercapto-2-methyl-phenoxy)-acetic acid methyl ester
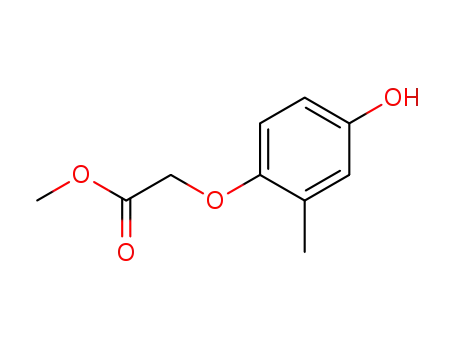
methyl (4-hydroxy-2-methylphenoxy)acetate
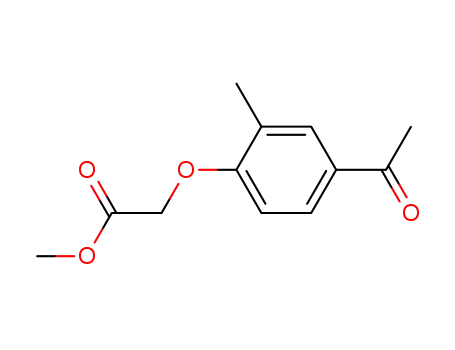
(4-acetyl-2-methylphenoxy)acetic acid methyl ester