CasNo: 22801-44-1
Molecular Formula: C15H22N2O
|
Originator |
Carboraine,Winthrop,US,1960 |
|
Uses |
Mepivacaine is a local anesthetic medication used in the United States for various medical purposes. It is commonly administered by healthcare professionals, particularly in dentistry and minor surgical procedures, to provide temporary and localized pain relief by blocking nerve signals in the targeted area. |
|
Manufacturing Process |
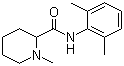
Ethyl magnesium bromide is prepared in the usual way by reacting 185 parts by weight of ethyl bromide in 800 parts of anhydrous ether with 37 parts by weight of magnesium turnings. Under vigorous stirring 121 parts of 2,6- dimethyl aniline are added at a rate depending on the vigor of the gas evaporation. The isoamyl alcohol solution is evaporated to dryness, the product dissolved in dilute hydrochloric acid, treated with charcoal and reprecipitated with NaOH. N-methylpipecolic acid 2,6-dimethyl anilide is obtained in crystalline form. |
|
Therapeutic Function |
Local anesthetic |
|
General Description |
Mepivacaine hydrochloride is available in 1% to 3% solutionsand is indicated for infiltration anesthesia, dental procedures,peripheral nerve block, or epidural block. The primary metabolic productsare the N-demethylated metabolite and the 3 and 4 phenolicmetabolites excreted as their glucuronide conjugates. |
|
Clinical Use |
The pharmacological and toxicological profile of mepivacaine is quite similar to that of lidocaine, except that mepivacaine has a slightly longer duration of action and lacks the vasodilator activity of lidocaine. For this reason, it serves as an alternate choice for lidocaine when addition of epinephrine is not recommended in patients with hypertensive vascular disease. |
|
Metabolism |
Mepivacaine undergoes extensive hepatic metabolism catalyzed by CYP1A2, with only a small percentage of the administered dosage (<10%) being excreted unchanged in the urine. The major metabolic biotransformations of mepivacaine are N-dealkylation (to give the N-demethylated compound 2′,6′-pipecoloxylidide) and aromatic hydroxylations. These metabolites are excreted as their corresponding glucuronides. |
InChI:InChI=1/C15H22N2O/c1-11-7-6-8-12(2)14(11)16-15(18)13-9-4-5-10-17(13)3/h6-8,13H,4-5,9-10H2,1-3H3,(H,16,18)
A novel and enantioselective synthesis o...
Herein we report a convenient, fast, and...
The results of this study indicate that the primary intraosseous injection of 2% lidocaine with 1:100,000 epinephrine is more successful and results in a longer duration of pulpal anesthesia as compared with 3% mepivacaine in noninflamed mandibular first molars. Most subjects reported no or mild pain during perforation and injection.
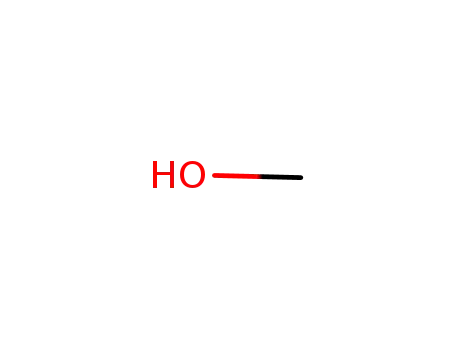
methanol

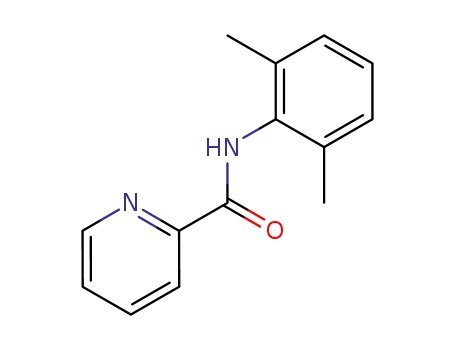
2-pyridine-carboxamide-N-(2,6-dimethylphenyl)

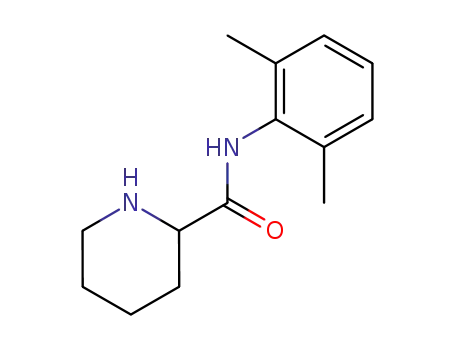
2',6'-pipecoloxylidide

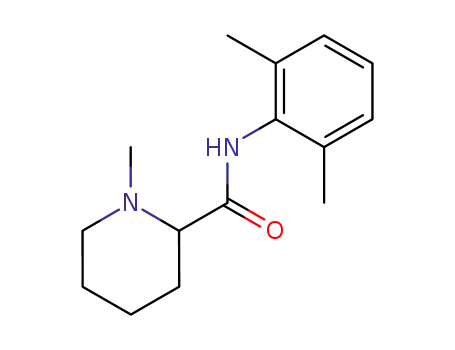
mepivacaine
| Conditions | Yield |
|---|---|
|
With palladium 10% on activated carbon; hydrogen; acetic acid; at 70 ℃; under 37503.8 Torr; Temperature;
|
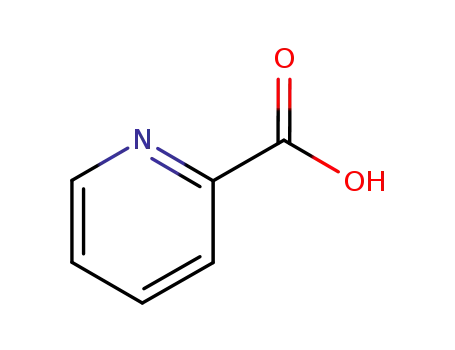
2-Picolinic acid


2',6'-pipecoloxylidide


mepivacaine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: trichlorophosphate / acetonitrile / 0.08 h / 150 °C / Microwave irradiation
2: acetic acid; palladium 10% on activated carbon; hydrogen / 70 °C / 37503.8 Torr
With palladium 10% on activated carbon; hydrogen; acetic acid; trichlorophosphate; In acetonitrile;
|