CasNo: 15883-20-2
Molecular Formula: C14H20N2O
Appearance: Off-white to light yellow powder
|
Chemical Properties |
White to Off-White |
|
Uses |
A major metabolite of Bupivacaine |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
An integrated process for the chiral sep...
Two series of ropivacaine analogs (4a–4q...
The invention discloses a preparation me...
Herein we report a convenient, fast, and...
The invention relates to the compounds o...
![[Cbz-piperidine-2-carboxylic acid (2,6-dimethyl-phenyl)-amide]](/upload/2023/8/7795468d-aba8-4f61-a513-f78ddf61488c.png)
[Cbz-piperidine-2-carboxylic acid (2,6-dimethyl-phenyl)-amide]

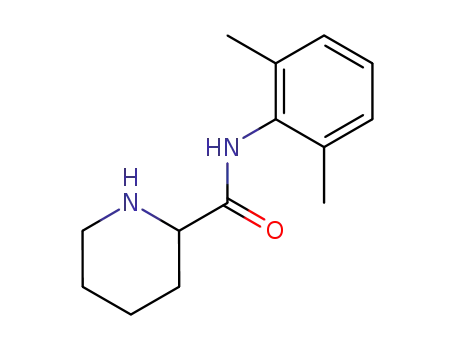
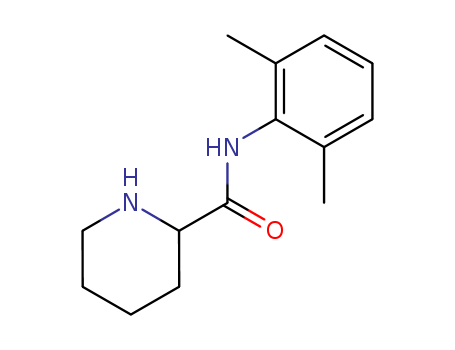
2',6'-pipecoloxylidide
| Conditions | Yield |
|---|---|
|
With
hydrogen;
palladium on activated carbon;
In
methanol;
for 0.5h;
|
92% |
|
palladium-carbon;
In
methanol;
|
92% |
|
With
palladium on activated charcoal; hydrogen;
In
methanol;
at 50 ℃;
for 0.5h;
Autoclave;
|
92.1% |
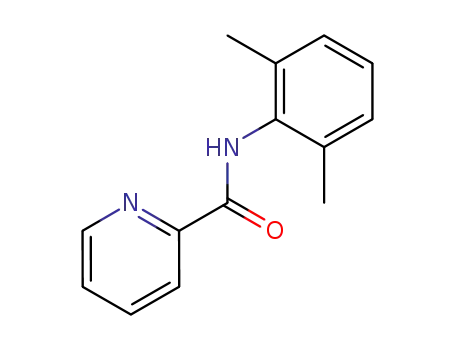
2-pyridine-carboxamide-N-(2,6-dimethylphenyl)


2',6'-pipecoloxylidide
| Conditions | Yield |
|---|---|
|
With
hydrogen; nickel; acetic acid;
In
methanol;
at 50 ℃;
under 13446.2 Torr;
Product distribution / selectivity;
|
72% |
|
With
hydrogen; acetic acid;
In
methanol;
at 50 ℃;
under 13446.2 Torr;
Sealed tube;
|
72% |
|
With
hydrogen; acetic acid;
In
methanol;
at 50 ℃;
under 13446.2 Torr;
|
72% |
|
With
hydrogenchloride; platinum;
Hydrogenation;
|
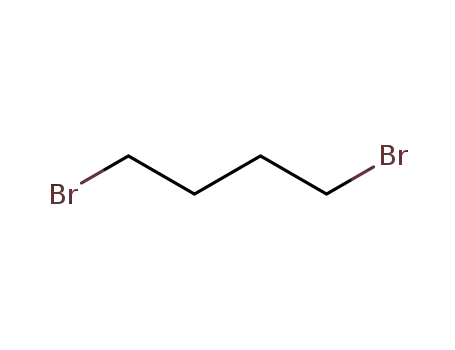
1,4-dibromo-butane
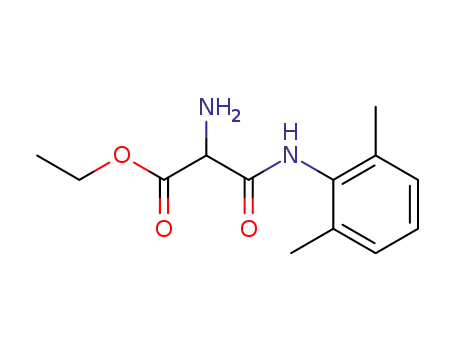
2-amino-N-(2,6-dimethyl-phenyl)-malonamic acid ethyl ester

2-pyridine-carboxamide-N-(2,6-dimethylphenyl)
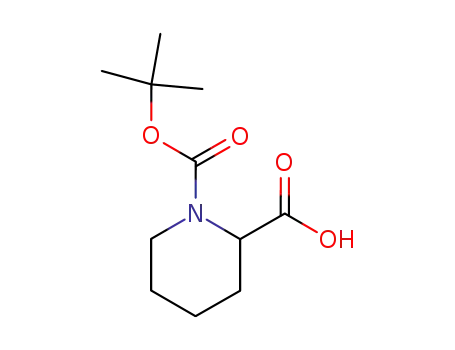
1-(tert-butoxycarbonyl)piperidine-2-carboxylic acid
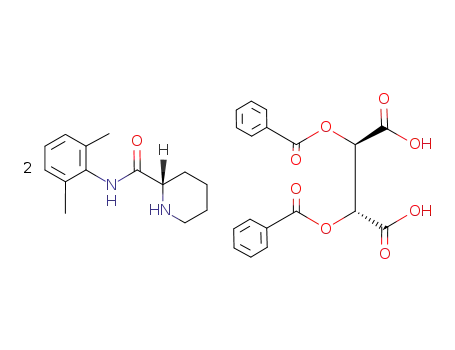
(S)-dibenzoyl-2-pipecolinoxylidide-L-tartrate
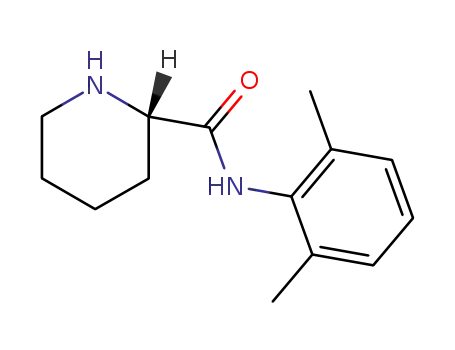
(S)-2',6'-pipecoloxylidide
(R,S)-N-(2,6-dimethylphenyl)piperidinium-2-carboxamide bis-(trifluoromethylsulfonyl)imide