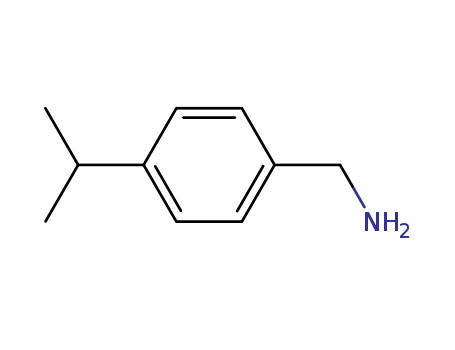
CasNo: 4395-73-7
Molecular Formula: C10H15N
|
Chemical Properties |
Colorless liquid |
| Uses | Isopropylbenzylamine is primarily known for its misuse as a cutting agent for illegal drugs, particularly methamphetamine. It resembles methamphetamine structurally but does not possess the same psychoactive properties. Due to its similarity to methamphetamine, isopropylbenzylamine has been illicitly added to methamphetamine to increase its volume and weight, making it more profitable for illicit drug manufacturers and sellers. |
InChI:InChI=1/C10H15N/c1-8(2)10-5-3-9(7-11)4-6-10/h3-6,8H,7,11H2,1-2H3
The yield of 2c obtained from ISOPROPYLBENZYLAMINE was lower in aqueous acetonitrile than in aqueous methanol (much gummy material was produced in this reaction)...
Arenes with various alkyl side-chains we...
The aerobic photochemical oxidation of benzylamine was carried out on the ternary oxides CuWO4 and BiVO4 as a test proton-coupled-electron-transfer reaction in acetonitrile. Both oxides give the coupled imine product, N-benzylidenebenzylamine, in near quantitative (98–99%) yield, with rate constants of 0.34 h−1 g−1 and 0.70 h−1 g−1 for CuWO4 and BiVO4, respectively.
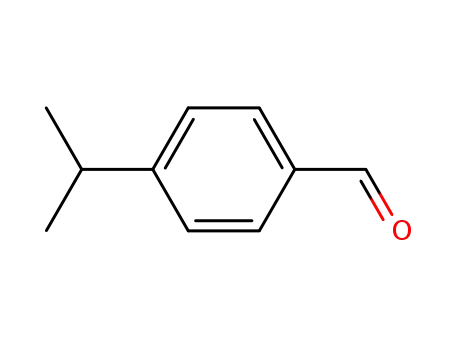
(4-isopropylbenzaldehyde)

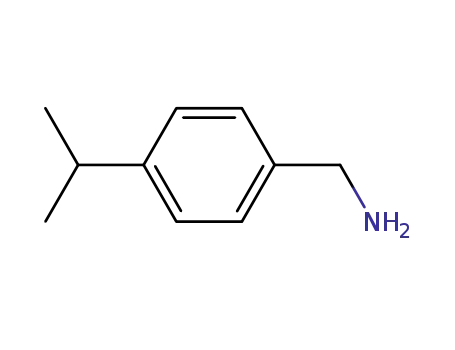
4-isopropylbenzylamine

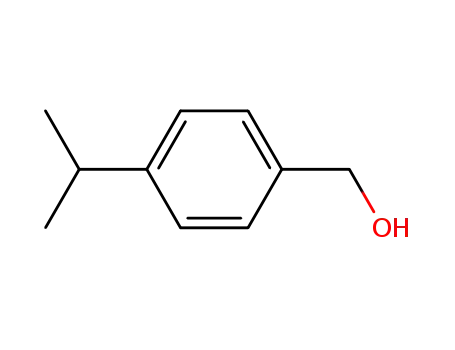
cuminol
| Conditions | Yield |
|---|---|
|
With ammonia; nickel; at 60 ℃; under 66195.7 Torr; Hydrogenation;
|
1-isopropyl-4-methylamino-2,5-cyclohexadiene-1-carboxylic acid


4-isopropylbenzylamine
| Conditions | Yield |
|---|---|
|
With chlorosulfonic acid; In dichloromethane; at 0 ℃; for 0.166667h;
|
76% |