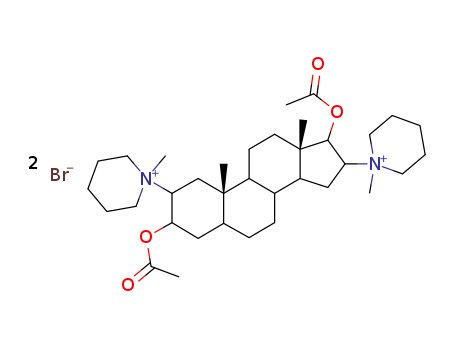
CasNo: 15500-66-0
Molecular Formula: C35H60Br2N2O4
Appearance: White powder
|
Originator |
Pavulon,Organon-Teknika ,UK,1968 |
|
Uses |
Pancuronium bromide, the pioneering steroid compound with a bisquaternary amine structure, was introduced to clinical use in 1964 by Savege and Hewitt. Its recommended intubation dose is 0.1 mg per kilogram of body weight, reaching peak effect within 3-4 minutes. Pancuronium's clinical action duration is notably long, particularly in the presence of potent inhalational agents or renal impairment, as 60% of the administered dose is excreted unchanged via the kidneys. Hepatic deacetylation occurs, yielding metabolites with neuromuscular blocking properties. Pancuronium bromide (Pavulon) is an artificial bisquaternary substance characterized by a steroid nucleus, denoted by the suffix "-curonium." It possesses approximately five times the potency of d-tubocurarine. Unlike d-tubocurarine, it does not elicit histamine release or block ganglionic transmission. Its onset of action is moderately slow (2.9 minutes), with a duration of action comparable to d-tubocurarine (110 minutes). Both pancuronium and its metabolite are excreted in urine. |
|
Brand name |
Pavulon (Organon). |
|
Therapeutic Function |
Muscle relaxant |
|
Biological Activity |
Nicotine (neuromuscular) antagonist. Skeletal muscle relaxant. |
|
Biochem/physiol Actions |
Aminosteroidal neuromuscular blocking agent; skeletal muscle relaxant |
|
Clinical Use |
Pancuronium bromide, a bisquaternary amine and the first steroid NMBA used clinically, was developed by Savege and Hewi and marketed in 1964. Unlike some other agents, pancuronium does not induce histamine release. However, it does possess direct vagolytic and sympathomimetic effects that can lead to tachycardia and hypertension. Pancuronium mildly inhibits plasma cholinesterase, potentially intensifying the effects of drugs metabolized by this enzyme, such as suxamethonium and mivacurium. As indicated, the principal use of pancuronium bromideis as an adjunct to anesthesia, to induce relaxation of skeletalmuscle, but it is also used to facilitate the managementof patients undergoing mechanical ventilation. Only experiencedclinicians equipped with facilities for applyingartificial respiration should administer it, and the dosageshould be adjusted and controlled carefully. |
InChI:InChI=1/C35H60N2O4.2BrH/c1-24(38)40-32-21-26-13-14-27-28(35(26,4)23-31(32)37(6)19-11-8-12-20-37)15-16-34(3)29(27)22-30(33(34)41-25(2)39)36(5)17-9-7-10-18-36;;/h26-33H,7-23H2,1-6H3;2*1H/q+2;;/p-2/t26?,27?,28?,29?,30-,31-,32-,33-,34-,35-;;/m0../s1
The impact of a novel steroid compound, pancuronium bromide (NA97), on the myoneural junction in humans has been examined. The drug was found to induce myoneural blockade, which was subsequently reversed with neostigmine. A 2-mg dose of pancuronium bromide elicited a level of blockade comparable in intensity and duration to that induced by 10–15 mg of tubocurarine. Electromyography revealed a swift decline in tetanus followed by post-tetanic facilitation. These observations suggest that the blockade is of the non-depolarizing or curaniform type. Upon intravenous administration of the drug, no alterations in pulse rate or systolic blood pressure were noted. Further exploration of the effects of pancuronium bromide in humans appears warranted.
This paper documents the cases of 12 patients treated over a 3-year span (from July 1, 1980, to July 1, 1983) who underwent artificial ventilation and received the muscle relaxant pancuronium bromide (Pavulon) for durations exceeding 6 days. Subsequent to discontinuing this medication, these patients developed severe tetraparesis characterized by areflexia, occasionally accompanied by impairments in the extraocular and facial muscles, as well as diffuse muscular atrophy, all without sensory deficits. Seven patients achieved complete recovery within 2-5 months, two experienced partial recovery, and three succumbed to their underlying illnesses. It is postulated that these neuromuscular complications stemmed from extended, high-dosage Pavulon treatment in conjunction with renal and hepatic dysfunction and/or the administration of aminoglycosides.
methyl bromide

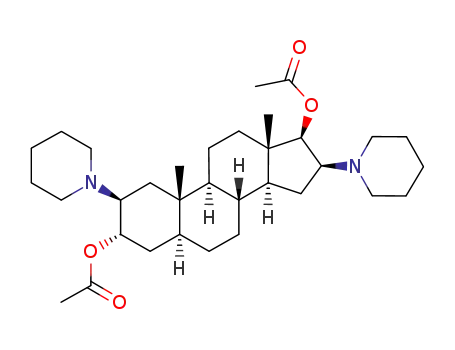
(2β,3α,5α,16β,17β)-2,16-bispiperidino-3,17-diacetoxy-androstane

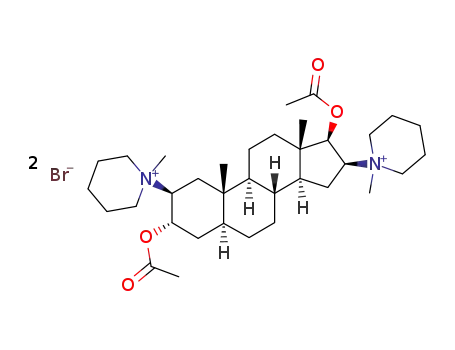
pancuronium bromide
| Conditions | Yield |
|---|---|
|
In acetonitrile; at 40 ℃; for 96h;
|
62% |
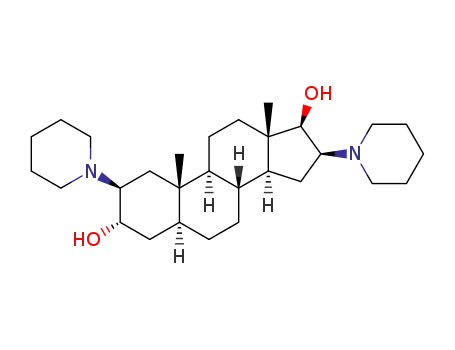
2,16-di(piperidin-1-yl)androstane-3,17-diol


pancuronium bromide
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: acetic acid / 6 h / 100 - 110 °C
2: acetonitrile / 96 h / 40 °C
With acetic acid; In acetonitrile;
|
|
|
Multi-step reaction with 2 steps
1: acetic acid / 6 h / 100 - 110 °C
2: acetonitrile / 96 h / 40 °C
With acetic acid; In acetonitrile;
|