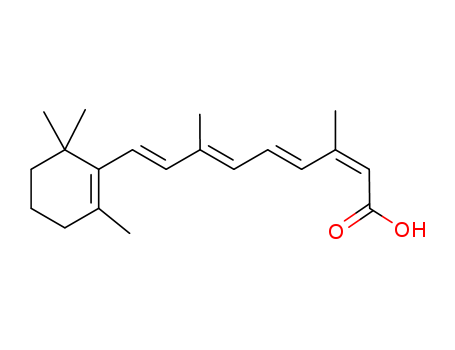
CasNo: 4759-48-2
Molecular Formula: C20H28O2
Appearance: yellowish crystalline powder
Isotretinoin is classified as a vitamin A derivative, and it exhibits a rapid and potent ability to inhibit the proliferation and differentiation of skin gland cells. This drug is particularly effective in treating severe nodular cystic acne. Isotretinoin boasts high gastrointestinal absorption but is not suitable for topical application. Its primary purpose is to address cases of severe acne that do not respond to other medications. Additionally, it shows efficacy in treating cystic acne, various types of acne, rosacea, ichthyosis, follicular keratosis, pityriasis red hair, and other dermatological conditions.
Isotretinoin falls under the category of first-generation vitamin A acids, serving as a stereoisomer of all-trans vitamin A acid. When administered orally, it exerts a pronounced anti-sebum effect, particularly beneficial for severe acne. This mechanism operates through several pathways:
InChI:InChI=1/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/p-1/b9-6+,12-11+,15-8+,16-14-
Isotretinoin, also known as 13-cis-retinoic acid, has found its place as a valuable retinoid for the treatment of numerous dermatologic conditions, often achieving remarkable outcomes. Despite its effectiveness in various skin disorders, it's essential to note that oral retinoids come with potential side effects and toxicities, necessitating vigilant oversight by experienced medical professionals. The utilization of oral retinoids in clinical practice continues to broaden, extending beyond the realm of dermatology, reflecting their growing significance in medicine.
Isotretinoin, also known as 13-cis-retinoic acid, is extensively utilized for treating severe acne, disorders related to skin conification, psoriasis, and skin cancer prevention. As a member of the retinoid family, it encompasses a broad range of potential side effects, spanning reproductive, cutaneous, ocular, neurological, musculoskeletal, and hepatic issues. When patients can withstand these side effects, it emerges as a highly effective treatment option. This article delves into both the most prevalent and the most concerning side effects, while also exploring strategies for healthcare providers and patients to effectively manage them, thus enabling the beneficial use of isotretinoin treatment.
![[(2Z,4E)-3-methyl-5-(2,6,6-trimethylcyclohex-1-en-1-yl)penta-2,4-dienyl]triphenylphosphonium chloride](/upload/2023/8/723cd9e5-44a3-4edc-872b-dc93cdc9df47.png)
[(2Z,4E)-3-methyl-5-(2,6,6-trimethylcyclohex-1-en-1-yl)penta-2,4-dienyl]triphenylphosphonium chloride

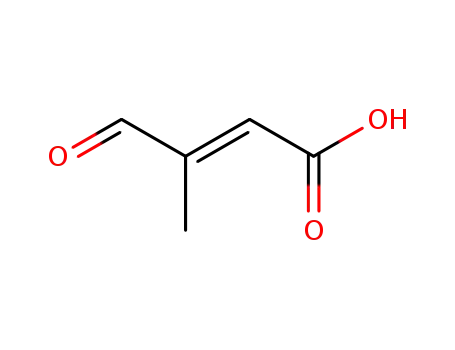
trans-β-formyl crotonic acid

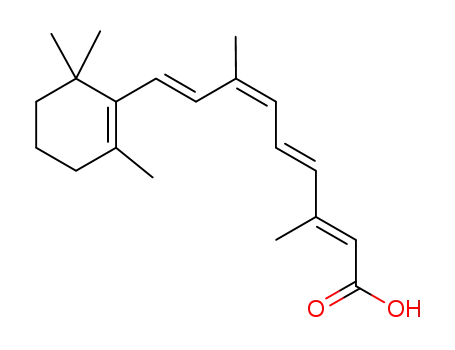
9-cis-retinoic acid
| Conditions | Yield |
|---|---|
|
[(2Z,4E)-3-methyl-5-(2,6,6-trimethylcyclohex-1-en-1-yl)penta-2,4-dienyl]triphenylphosphonium chloride; trans-β-formyl crotonic acid; With sodium hydroxide; In isopropyl alcohol; at -50 - -40 ℃; for 24h; Inert atmosphere;
With hydrogenchloride; palladium diacetate; In isopropyl alcohol; at 80 ℃; pH=8-9; Inert atmosphere;
|
82.3% |
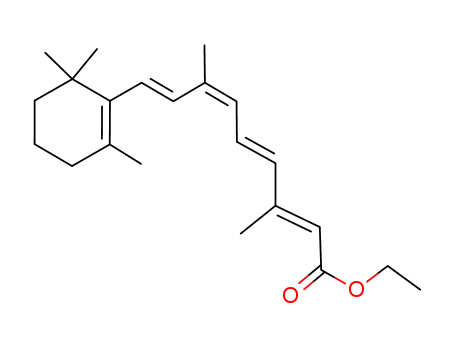
ethyl (2E,4E,6Z,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraenoate


9-cis-retinoic acid
| Conditions | Yield |
|---|---|
|
With potassium hydroxide; In ethanol;
|
100% |
|
With potassium hydroxide; In ethanol; at 50 ℃;
|
98% |
|
With potassium hydroxide; In ethanol; water; at 70 ℃;
|
91% |
|
With potassium hydroxide; In ethanol; at 70 ℃;
|
91% |
|
With sodium hydroxide; In methanol; at 50 ℃; for 0.5h;
|
74% |
|
With sodium hydroxide; In methanol; at 50 ℃;
|
70% |
|
With sodium hydroxide; In ethanol; Yield given; Heating;
|
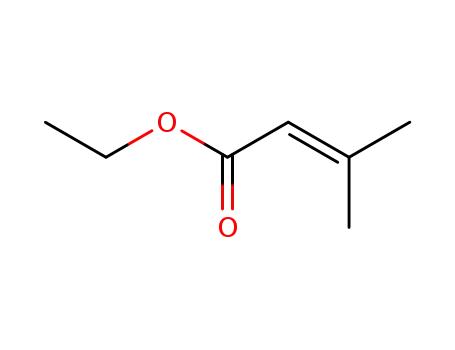
ethyl 3-methylbut-2-enoate
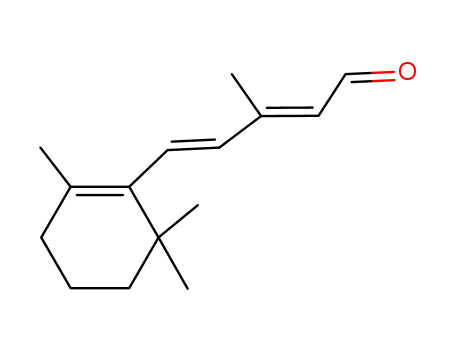
(2E,4E)-3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienal
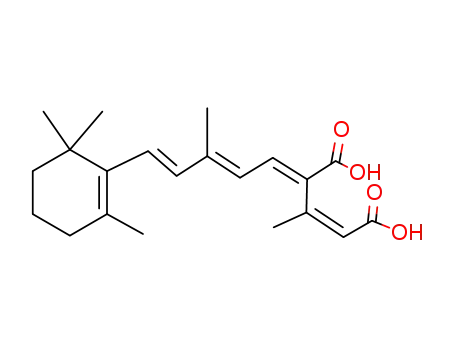
11-cis,13-cis-12-carboxyretinoic acid
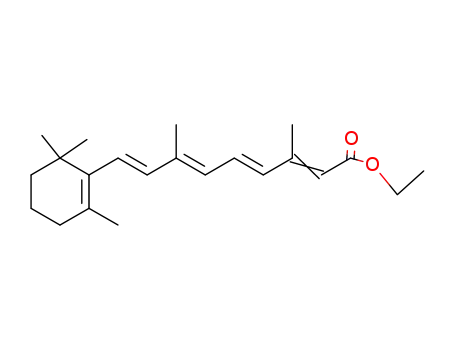
ethyl retinoate
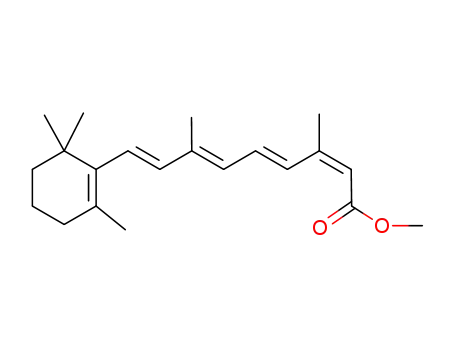
(2Z,4E,6E,8E)-methyl 3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraenoate
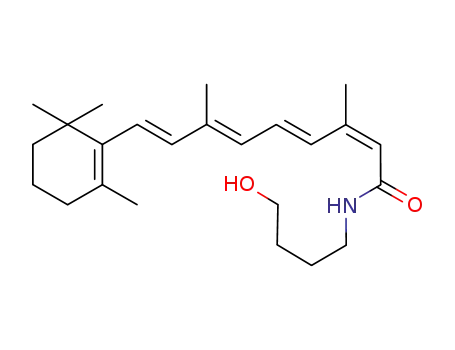
13-cis-N-(4-Hydroxybutyl)retinamide
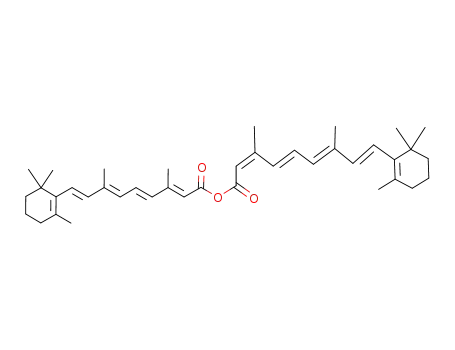
all-(E)-retinoic and 13-(Z)-retinoic anhydride
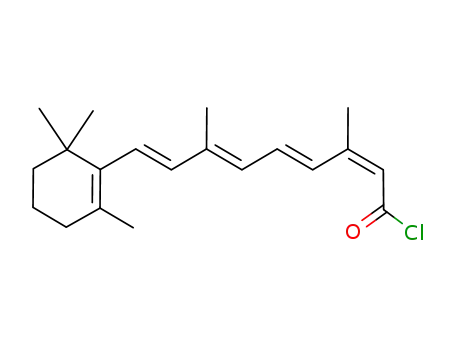
13-cis-retinoyl chloride